Bad Batch = Good Reporters?
Why there were so many Adverse event reports for some Covid-19 vaccine batches
Gold news: There are no bad batches
Bad news: That makes it much worse
This is a short summary of my findings in VAERS data analyses of Covid-19 vaccine adverse event reports. You can find the list of detailed articles at the end.
Batches and Reporting Rates
Event -> Reporting Rate -> Adverse Event Report
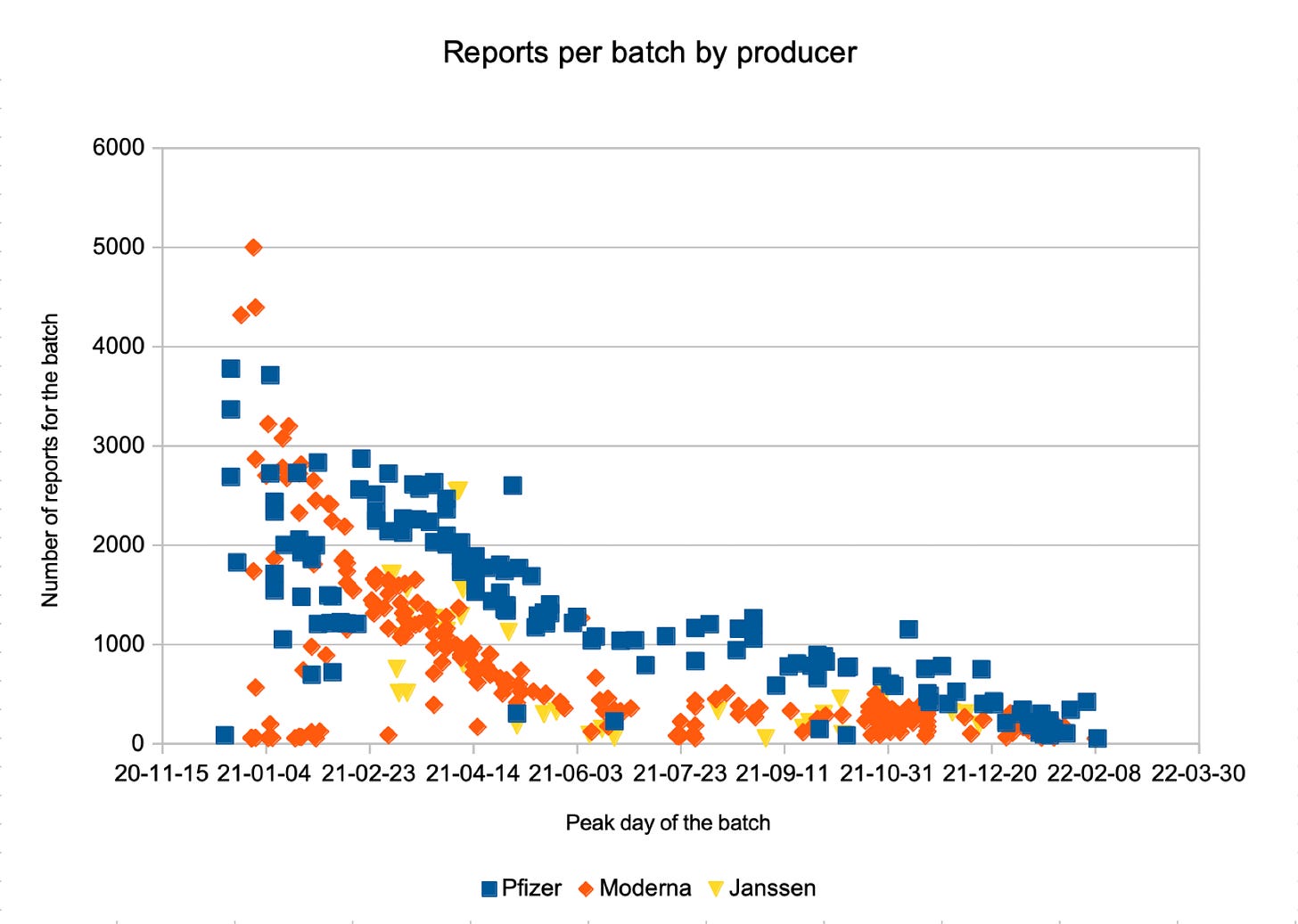
It is a well-known fact that there are big differences in the number of adverse event reports per vaccine batch, which led to the conclusion that there were batches of particularly bad quality (Bad Batches).
In fact, the real cause of these differences are characteristics of the vaccinated persons and the reporters, for which I show evidence in my statistical analyses.
In consequence, the presumably „bad“ batches show the real order of magnitude of adverse events.
„Bad“ Batches
Impact of COVID-19 Vaccine Priority Groups
The biggest differences in adverse event report numbers are observed in the earliest Covid-19 vaccine batches since their primary recipients were COVID-19 Vaccine Priority Groups.
One of these groups was health care professionals which are likely to have caused far above-average reporting rates.
After the first three months, all vaccine batches have similar reporting characteristics
Plummeting Report Rates
Why date is the most important factor for report quality
In the first weeks, there were high numbers of VAERS reports per vaccination (reporting rates), but, starting with 1/1/2021, reporting rates plummeted by 50% within 18 days, and by a further 50% within the next 35 days.
This extremely fast decline raises suspect of external influence to reporting behavior (regime change?)
From Dec 2020 until early 2022, reporting rates have declined by a factor of 16 (94%) – for all types of events: mild, severe and death
Main Factors influencing Reporting Rates
They explain the majority of observed rates
The most important factors for the number of adverse events per batch are
Vaccine administration date
Age (for severe events)
Gender
Vaccination site
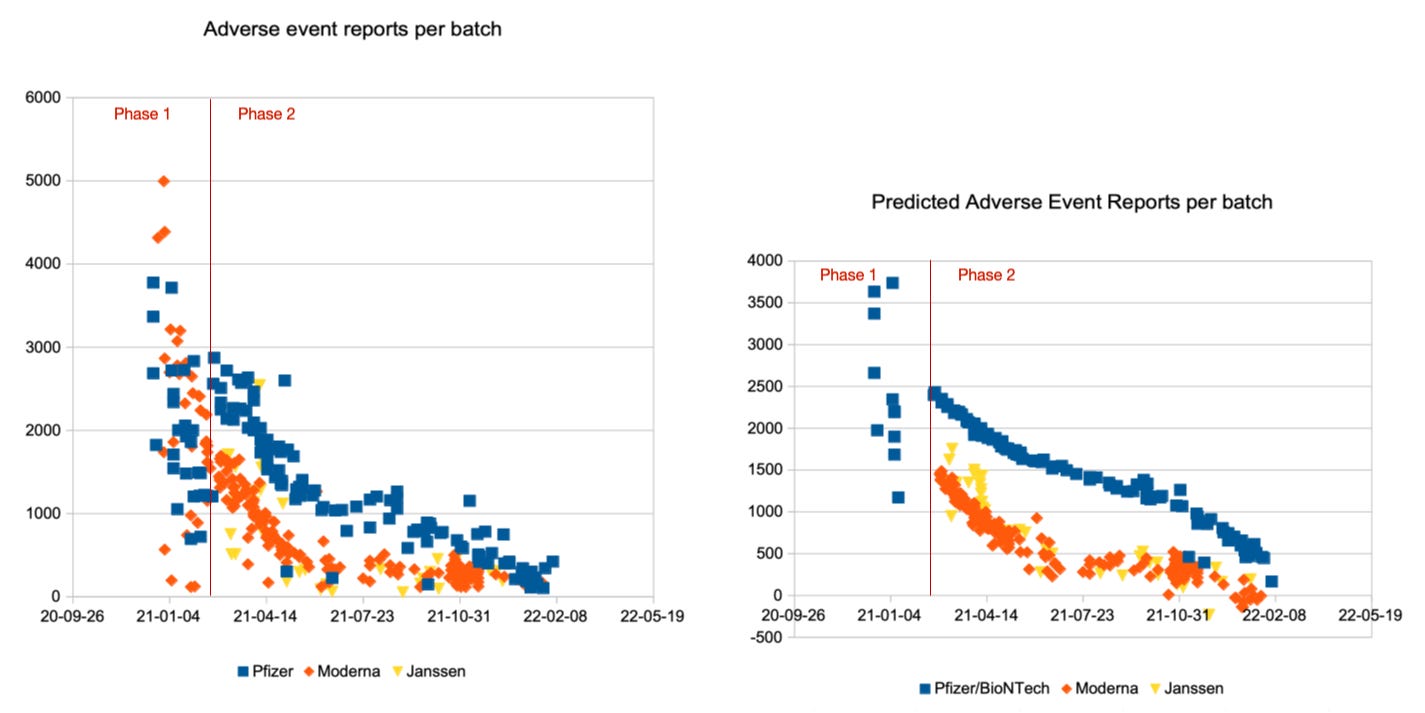
A multilinear regression over these factors provides very good predictions for the report counts of a batch, except for the first three months, in which priority groups dominated.
I was able to define linear formulas with the above input factors which calculate good estimates of adverse event counts and severe adverse event counts. For a visual impression of their prediction quality, see the comparison of reported adverse event counts on the left with the predicted counts on the right. First, in the upper row, for all adverse events, then, in the lower row, for the severe adverse events only. Pfizer/BioNTech batches are displayed with blue squares, Moderna batches with orange diamonds and Janssen batches with yellow triangles:
Overall, prediction quality looks pretty good, especially with regard to the simplistic linear approach, and make it very clear that the variety in adverse and severe adverse event counts per batch can mostly be explained without production issues of the manufacturers.
Consequences
The most-reported batches are not bad – they are the standard
Since not batch content but reporting makes the difference, the batches with the largest reported adverse event counts are the ones that most closely resemble the number of adverse events that actually occurred. They are the standard, not the exception!
Underreporting Rates
and estimates of the real-world numbers
A very conservative extrapolation of the most reported batches’ data yields an overall underreporting rate of factor 7 with
160,000 severe adverse events (life-threatening symptoms, permanent disability, congenital damage, abortion or hospitalization)
42,000 deaths
For 255 million vaccinated people in the US, this estimate means that one out of 6,000 people died after vaccination
Further reading
Where to find the details and how to stay informed
This is just a short summary of my findings, they are explained in detail in a series of three online articles:
A follow-up article on this topic is:
About Me
Because of strong polarization in the discussion of vaccine safety, I prefer to stay anonymous as of now and use the pseudonym Leonard Frey. Feedback is welcome and you can contact me by email: leonard_frey@yahoo.com
You can stay informed about my newest findings by subscribing to my blog at Substack: https://leonardfreyen.substack.com/ in English or https://leonardfrey.substack.com/ in German





I acquired batch information from Australia via a Freedom of Information request and analysed it here https://accaen.substack.com/p/batch-data-from-covid-19-vaccines
The Australian data set is very limited compared with VAERS, but I think it implies different reporting rates between health professionals and the public.